WACKER’s α-Silane Technology
With the development of the α-silane technology, WACKER scientists made the transition to the silane-terminated-polymers market in 2006. In doing so, they were able to redefine formulation strategies, because the reactivity of these systems made it possible to break away from hitherto firmly established pathways. The omission of metal salts that are harmful to health resulted in a significant improvement in the consumer friendliness of the formulations and prevention of side effects such as undesired side reactions in the product. At the same time, sustainability benefits typical of SMPs were retained. What was the key to these successes?

SMP Technology Basics
Silane-terminated polymers have been used as binders for sealants, adhesives and coatings for over 30 years. There are a number of reasons for this. They can, for example, be formulated into one-component, isocyanate-free sales products without the use of solvents thanks to their low viscosity. It is beneficial that the products cure without bubbles even under unfavorable weather conditions. Nowadays, many wood-flooring adhesives are based on silane-terminated polymers, as are several assembly adhesives and exterior waterproofing systems. In these kinds of applications, the elasticity of the cured materials and their wide adhesion spectrum are of particular importance.
Silane-terminated polymers are produced by coupling an organic polymer with an organofunctional silane. The organofunctional silane itself has two faces: each one of these molecules combines the functionality of a reactive organic group with the inorganic functionality of a silane.
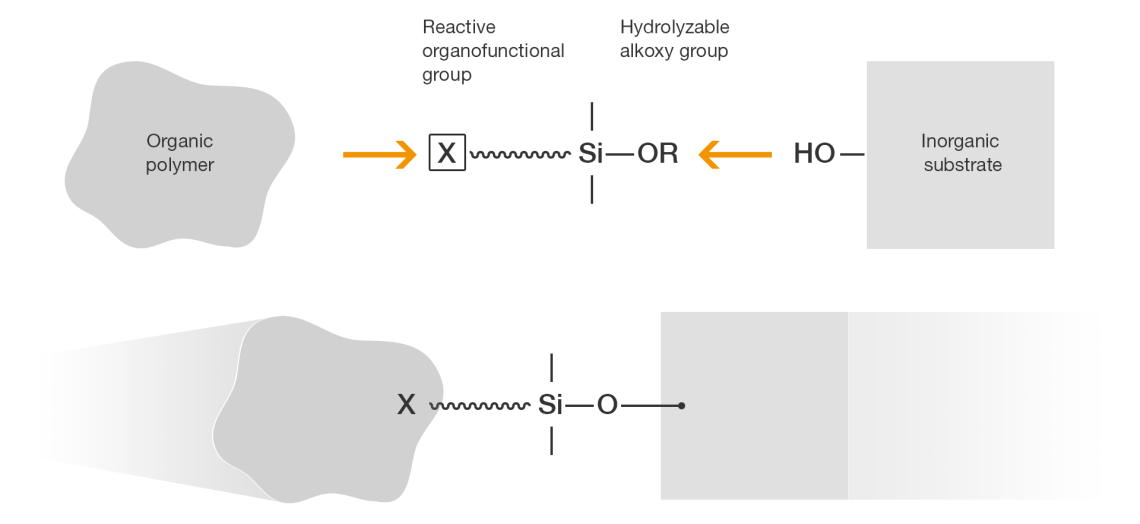
Here, the reactive organofunctional group X can be varied, depending on the nature of the organic polymer. Silanes may contain amino, epoxy, glycidoxy, mercapto, sulfido, iscocyanato, methacryloxy or vinyl groups. The bond to the inorganic substrate is formed on the other side via hydrolyzable functional groups, OR. Predominantly methoxy and ethoxy substituents are used to form these. The silane’s alkoxy groups hydrolyze as soon as they come into contact with atmospheric moisture.
The hydrolysis yields reactive silanols. These then either condense to form polymeric siloxane networks or they react with the inorganic substrate.
The Difference Between α- and γ-Silane-Terminated Polymers
All commercially available silane-modified polymers (SMPs) contain alkoxysilyl groups and are thus able to crosslink in the presence of moisture. They differ in their organic backbone, the connection of the silyl groups to this polymer skeleton and the distance between the silyl groups and the backbone. In the case of silane-terminated polyethers (GENIOSIL® STP-E, referred to as STP-E hereinafter), the silyl groups are coupled to the ends of the polyether backbone via an alkylene unit and a urethane group.

The alkylene unit can either be a methylene group, in which case it is an α-silane-terminated polyether, or a propylene group, in which case it is a γ-silane-terminated polyether.
The α-Effect in a Nutshell

Structure of an α-silane
The length of the alkylene unit influences the reactivity of the alkoxysilyl groups toward moisture: formulations of α-silane-terminated polyethers cure rapidly without the need for a tin catalyst; the presence of a catalytic primary amine is sufficient.

Structure of a γ-silane
In the case of γ-silane-terminated polyethers, on the other hand, a tin catalyst or a strong base such as 1,8-diazabicycloundecene (DBU) is always required.
Both types of silane-terminated polyethers have specific advantages. γ-Silane technology yields products that feature particularly high elastic recovery. In the case of α-silane technology, the wide formulation latitude that stems from the absence of a tin catalyst is paramount. Ester-based plasticizers can be used, for example, since there is no risk of ester hydrolysis without a tin catalyst. The shelf life of the formulations is also longer. Furthermore, the absence of organotin compounds is beneficial for environmental and health reasons.



