Our system has noticed that you are based in United States, but the current country setting is Uganda. Do you still want to change your country?
Expert Plasmid DNA Production
Wacker Biotech is your go-to CDMO expert for the production of plasmid DNA (pDNA). pDNA is the critical starting material for advanced mRNA therapies and vaccines, gene or gene-modified cell therapies, and vector-delivered therapies (e.g., AAV and LV). There are some applications, such as DNA vaccines, using pDNA as the direct therapeutic itself.
Contact uspDNA Innovation and Experience
With over 100 GMP pDNA batches produced, our San Diego center of excellence has been at the forefront of plasmid development and production for all clinical study stages, including Phase III, since 2003. We also offer these capabilities in Halle, Germany.
Our dedicated team of approximately 30 nucleic acid research scientists in Munich continuously innovates new production methodologies and technologies. Our proprietary plug-and-play PLASMITEC® platform streamlines pDNA manufacturing with efficient fermentation and downstream purification, ensuring high-quality production without compromise.
pDNA Capabilities
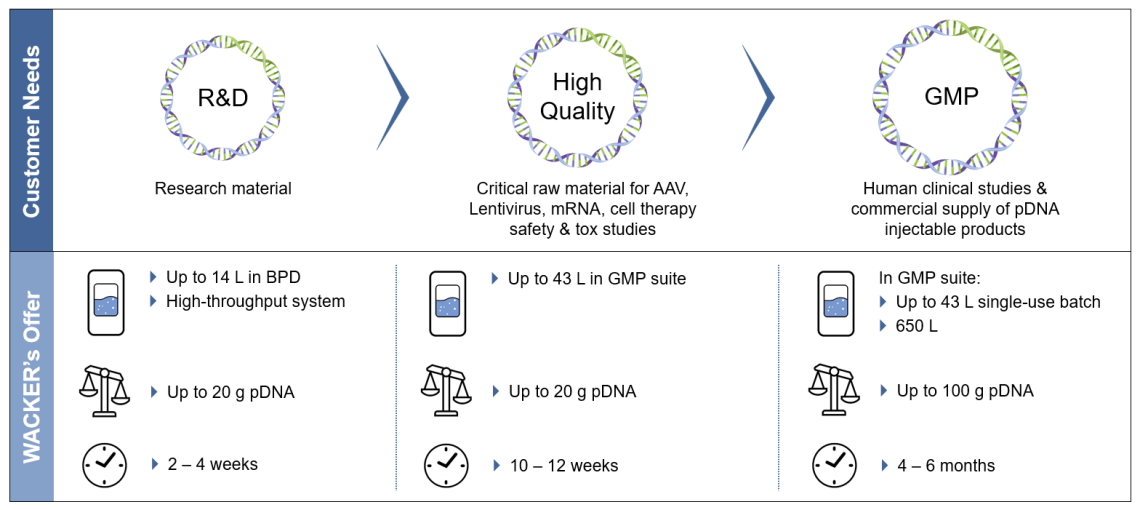
- Various Quality Grades
Material supply for Research Grade, High Quality and GMP Quality. For Research Grade material, our high-throughput system can screen for many different plasmids simultaneously. - Strain Development
Generation and selection of the top-performing E.coli strains for supercoiled/linearized pDNA. - Development of Customized Processes
A dedicated process development lab to support feasibility, process development and optimization as well as comparability. - Technology Transfer
Flexible and experienced MSAT teams to accommodate your customized pDNA processes. - Analytical Capabilities
Comprehensive analytical portfolio for product release, cell-based assays and stability studies. - GMP Cell Banking
Master and working cell bank production. - GMP Manufacturing
Scalable pDNA production from 43 L to 1500 L scale including scalable lysis and dedicated lines for chromatographic purification for clinical trials or commercial supply. Learn more about our state-of-art pDNA facilities and manufacturing capabilities. Learn more about GMP Manufacturing at WACKER. - Plasmid DNA for mRNA
At its pDNA center of excellence in San Diego as well as its site in Halle, Wacker Biotech can produce template pDNA for mRNA IVT and mRNA-based therapeutics and vaccines. Click below to learn more. - Plasmid DNA for Viral Vectors
Through a partnership with Expression Manufacturing, Wacker Biotech offers viral vector capabilities. We provide the plasmids, while Expression provides the backbone technology and viral vector manufacturing. Click below to learn more.
Key Benefits of Wacker Biotech’s pDNA Services
- Extensive Track Record
Over 20 years of experience in manufacturing plasmids for all stages of clinical studies, with more than 100 GMP pDNA batches produced. - Scalability
Seamless transitioning from 43 L up to 1,500 L for maximum flexibility throughout your product’s lifecycle. - End-to-End Capabilities
Full manufacturing chain coverage for nucleic acid-based therapies, from pDNA to mRNA to LNP. - Innovation and Know-How
Novel plasmid designs, cutting-edge pDNA production technologies, scalable processes, and ongoing strain development. - Efficiency and Speed-to-Market
Our PLASMITEC® platform for efficiently producing complex plasmids, speeding project turnarounds, and streamlining regulatory filings.

Ready to Partner with Us?
Discover how Wacker Biotech can transform your biopharmaceutical production. Contact us today to learn more about our innovative solutions and how we can help you achieve your goals. Reach out to our team for more information.
Contact us
















